
Matthay KK, Reynolds CP, Seeger RC, et al.
#AUTOLOGOUS STEM CELL TRANSPLANT CURE TRIAL#
A randomized phase III trial of myeloablative autologous peripheral blood stem cell (PBSC) transplant (ASCT) for high-risk neuroblastoma (HR-NB) employing immunomagnetic purged (P) versus unpurged (UP) PBSC: a Children's Oncology Group study. Kreissman SG, Villablanca JG, Seeger RC, et al. Ladenstein R, Pötschger U, Pearson ADJ, et al., Busulfan and melphalan versus carboplatin, etoposide, and melphalan as high-dose chemotherapy for high-risk neuroblastoma (HR-NBL1/SIOPEN): an international, randomised, multi-arm, open-label, phase 3 trial. Stem cells can be stored safely without cryopreservation for up to 7 days.Īutologous stem cell transplant cryopreservation neuroblastoma plerixafor survival treatment. Treatment abandonment was observed in one (3%) patient.Īdministration of therapy in a disciplined time frame along with low-cost adaptations enables to manage high-risk neuroblastoma with low abandonment and an encouraging survival in LMIC. The 3-year overall and event-free survival was 41% and 39%, respectively. Two (5.7%) patients had ASCT-related mortality. The median viability of stem cells stored for 6 days (n = 28) was 93% (range: 88-99). Conditioning regimen included melphalan (n = 7), oral busulfan-melphalan (Bu/Mel n = 6), or intravenous Bu/Mel (n = 22). Rapid-COJEC was administered over a median duration of 80 days (interquartile range: 77, 83). Over 5 years 9 months, 35 patients with high-risk neuroblastoma were treated.

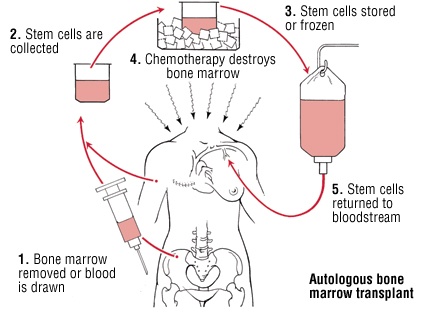
Adaptations incorporated to decrease cost, requirement for inpatient admission, infections, and faster engraftment included (a) optional outpatient administration for rapid-COJEC, (b) two sessions of stem-cell apheresis, (c) storing stem cells at 2-6☌ without cryopreservation for up to 7 days, (d) no central lines, (e) no antibacterial/antifungal/antiviral prophylaxis, (f) omitting formal assessment of cardiac/renal/pulmonary functions before ASCT, and (g) administration of pegylated granulocyte colony-stimulating factor on Day +4. Patients were treated on the backbone of the high-risk neuroblastoma study-1 of SIOP-Europe (HR-NBL1/SIOPEN) protocol with ASCT. We report a single-center outcome of high-risk neuroblastoma, with adaptations optimized for LMIC.
Management is intensive and multidisciplinary survival is often poor. The majority of patients in low- and middle-income countries (LMIC) are unable to receive optimal therapy, including autologous stem cell transplant (ASCT) for high-risk neuroblastoma.


 0 kommentar(er)
0 kommentar(er)
